Which cow genes contribute to better heat tolerance?

Scientists around the world are hard at work trying to determine which genes contribute to better heat tolerance in dairy cattle, and how to increase the number of cows with these genes or with increased expression of these genes – here we take a look at the latest.
One major project involves a gene-editing technique that allows the ‘slick gene’ to be introduced into newly-fertilized eggs from cows without the gene. This is being achieved by scientists at the Centre for Tropical Livestock Genetics and Health (CTLGH) at The Roslin Institute, University of Edinburgh, Scotland.
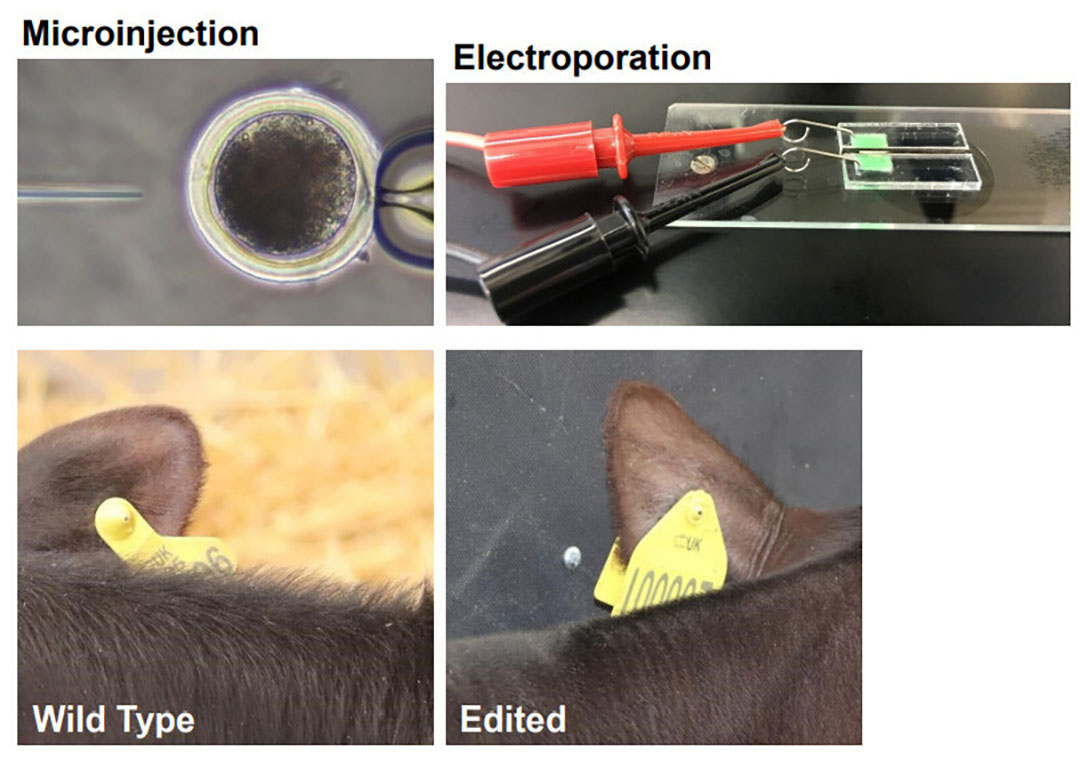
The modified embryos were transferred into surrogate host mother cows. Calves were born with modified coats, and there were no signs that the gene edit had any other impacts. The ‘slick gene’ coat allows cows to better handle heat through short and sleek hair that’s sometimes glossy.
The gene originated in Senepol cattle in the Caribbean Island of St. Croix. It is being used in breeding programmes in many countries.
The scientists note that inserting this gene using this gene editing technique has great potential to build up the cow populations better adapted to heat stress around the world, and that it would be especially useful in assisting small-holders in tropical countries.
That’s why colleagues of The Roslin Institute scientists at the International Livestock Research Institute (ILRI) in Nairobi, Kenya are being supported to use this gene edit approach in native cattle breeds there.
A look at the technique
To do this CRISPR gene edit, the scientists are using a technique that involves an electric field to assist with microinjection of DNA into a fertilized egg.
Dr Simon Lillico, core scientist at the University of Edinburgh, first notes that microinjection of DNA into an egg is an expensive and technically difficult procedure.
“The technique of electroporation (making pores with an electric field) has been around for many years for introducing DNA into cells, but only recently has it been used with zygotes,” he says. “There are 2 electrodes with conductive material in between where the zygote sits, and we have to use the correct level of electricity. We have to use a level low enough to not kill the zygote, but high enough so that you make very temporary holes in the zygote cell membrane so that DNA and proteins that are initially outside the cell will move in the electric field into the cell.”
CTLGH director, Dr Appolinaire Djikeng, notes that it took months, but a good success rate with DNA transfer and also relatively good survival of the zygote was found. “However, we want to develop a method of DNA insertion that is easier and cheaper in the future for both developed and less resourced laboratories,” he says.
The team did various heat tolerance tests on a number of cows derived from zygotes that received the gene edit. Tissue samples are also being tested to examine gene expression, specifically the prolactin signalling pathway that is involved in immunity, fertility, milk production and hair production.
“The tie between the slick coat and an improved ability to radiate heat is not yet proven,” says Lillico, “so we are looking at a variety of tissues to examine the prolactin pathway and to see if there are other signalling pathways involved in improved heat tolerance. We have no idea how many genes may be involved.”
The work of CTLGH with colleagues at ILRI on gene editing continues in relation to heat tolerance, humidity stress, disease resistance and more with cattle as well as poultry.
Future adoption
Global acceptance of gene editing is growing, notes Lillico. “There are clear legal frameworks and approved animal products in Argentina, Brazil, and Japan and the US,” he says. “There are gene-edited cattle in the US that do not have horns, and the team that achieved this is working with techniques similar to ours. I would think that widespread adoption of gene editing in agriculture will be first in the Americas, with much activity in the US, Argentina and Brazil.”
Lillico believes the demand for editing by breeding companies will be a major factor in broad adoption, as these are the players with the largest impact in terms of numbers and distribution.
He adds that “whilst it’s not yet legal in the UK for food production applications, there is certainly support for it from both scientists and the agri-food industry here.”
In 2021, scientists at the Roslin Institute welcomed moves by the UK government to review regulations around gene editing that followed a nationwide consultation. The UK is able to develop its own regulations in the wake of Brexit.
Djikeng notes that Kenya is a leading country in Africa for genetics and other areas of science, and there, recent clarifications have been made to differentiate the regulatory framework for gene-edited crops and livestock from that of transgenic organisms.
“With gene editing, we can do small edits in the genome that very rapidly accomplish improvements that would otherwise take 20 generations,” he says. “I am very excited about the potential adoption of this technology in Kenya, the UK and other places around the world.”
Australia: Narrowing down the genes
As in other regions and countries where it’s often hot and many cows are out grazing most of the year, genetics is an important heat mitigation strategy for Australian dairy farmers. Agriculture Victoria says that the tolerance tipping point for many cows seems to be in the low 20s in degrees Celsius and about 45% humidity.
Australia has had genomic breeding values for heat tolerance available for dairy farmers since 2017 through DataGene, an independent organisation responsible for driving genetic gain and herd improvement that is an initiative of Dairy Australia and the country’s dairy industry.

Millions of markers
“The research used a whole genome approach to search for genes that might be useful for predicting heat tolerance in cows – looking at around 15 million genetic markers on about 30,000 Australian Holstein dairy cows,” says an Agriculture Victoria spokesperson representing the project.
“We identified variations in the DNA around a number of genes that were associated with more heat-tolerant animals. Out of these, 6 were part of the nervous system that relate to how the animal reacts to its environment.”
A follow-up study was conducted to see if the genes also predicted Jersey heat tolerance, as the theory was that many of these gene ‘regions’ are highly conserved. However, it was found that although these special genes increased the accuracy of genomic prediction by about 5% units in Holsteins, the increases were less consistent in Jerseys.
Many genes interacting
A key finding is that many genes affect heat tolerance and variation, some of those genes also affect how productive the cow is.
As with the research at the University of Edinburgh, these results will help the Australian scientists understand the biological mechanisms important for heat tolerance.
Agriculture Victoria says a key focus of the ongoing research is to better understand how the genes involved interact with other important traits such as production, health and fertility and to develop more precise but affordable measures for heat tolerance.
The results will also be used in a new 5-year project to compare the newly-found genes with DNA from dairy cows across Australia to find new ways to identify the most heat-tolerant cows for breeding.
Researchers are developing a larger data pool than was used for the current version of the breeding value to help increase the accuracy of genomic prediction. By the end of 2023, Agriculture Victoria’s aim is to have finished the research part of the ‘potential new reference population for heat tolerance’ in Australian Holsteins and Jerseys, adding in sensitivity in somatic cell count (an indicator of udder health) as well as production traits that the team have already investigated.
Agriculture Victoria notes that there is scope to use other outcomes of heat events on dairy cows, such as changes to milk composition or even cow behaviour and physiology, to improve genomic predictions of heat tolerance.
Join 13,000+ subscribers
Subscribe to our newsletter to stay updated about all the need-to-know content in the dairy sector, two times a week.










